Research
A fundamental question in developmental biology is how cells communicate with one another and the environment. Receptor tyrosine kinases (RTKs) contribute to this communication by binding to growth factors at the cell surface and activating various intracellular signaling pathways to elicit responses with broad roles in developmental cellular processes. A subset of RTK families has been shown to regulate the activity of neural crest cells (NCCs) and the development of their derivatives in mammalian systems. NCCs are migratory, multipotent cells that play a critical role in vertebrate development. Cranial NCCs originate from the forebrain to the hindbrain and populate the frontonasal prominence and pharyngeal arches 1-4. These cells give rise to the bone and cartilage of the frontonasal skeleton, among other derivatives.
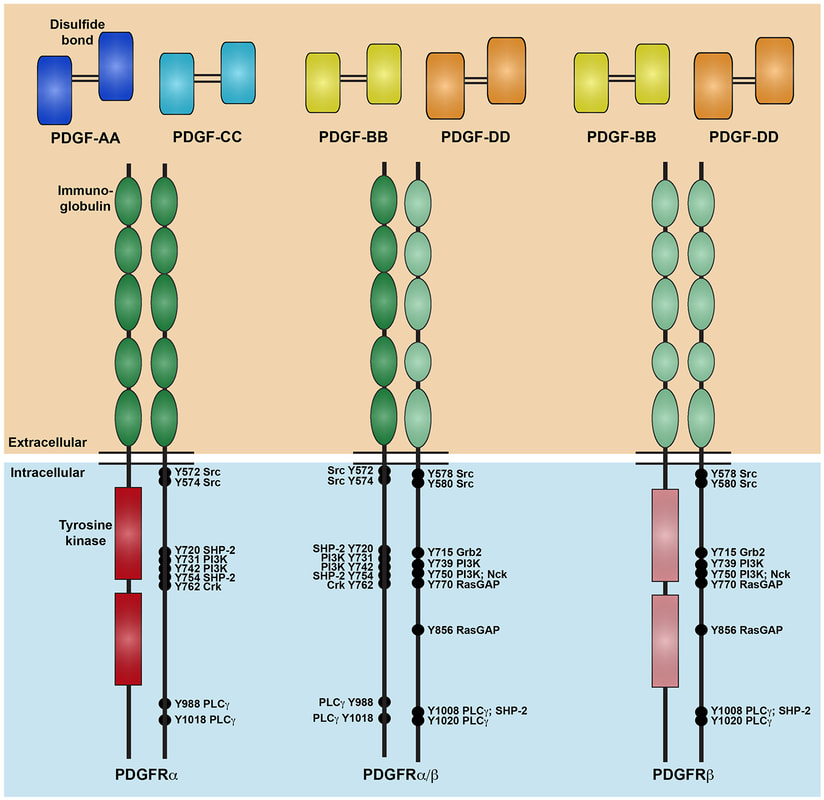
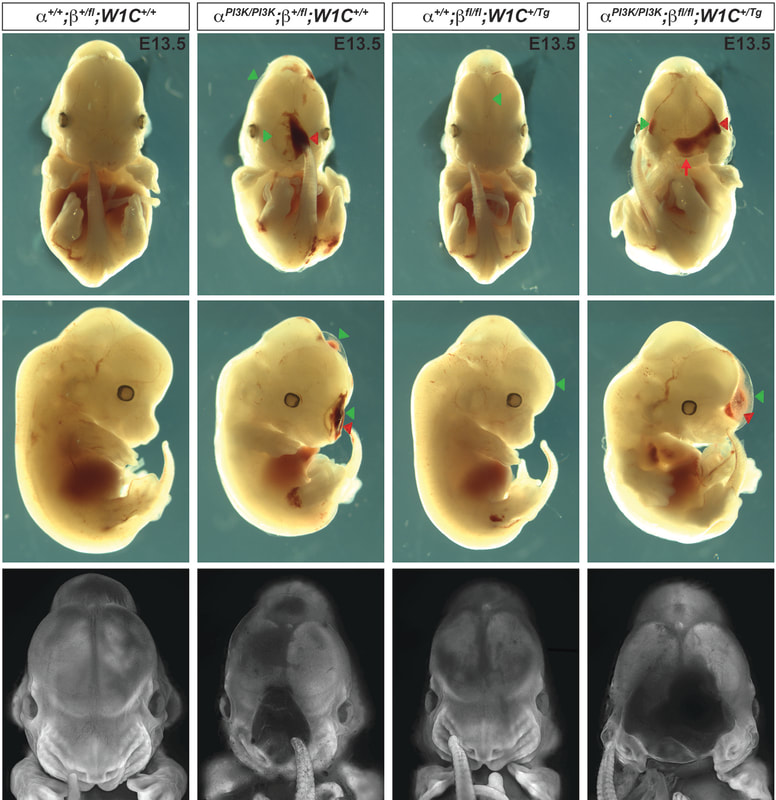
Our lab is focused on investigating the mechanism and function of signaling through a particular RTK family, the platelet-derived growth factor (PDGF) receptor family, in development of the cranial NCC-derived craniofacial skeleton. Craniofacial development is a complex morphogenetic process, disruptions in which result in highly prevalent human birth defects. Signaling through the PDGFRs plays a critical role in this process in both humans and mice. Pdgfra mutant mouse models display a range of craniofacial phenotypes such as midline clefting, subepidermal blebbing and hemorrhaging. Functional analysis of PDGFRalpha signaling during mouse development by our lab and others has revealed roles in promoting migration of cranial NCCs, proliferation of the NCC-derived craniofacial mesenchyme and osteoblast differentiation. We showed that alternative RNA splicing is an important mechanism of gene expression regulation downstream of PDGFRalpha signaling in the facial mesenchyme and identified the RNA-binding protein Srsf3 as a critical regulator of craniofacial development downstream of this pathway. We have demonstrated a role for the other RTK in this family, PDGFRbeta, in murine craniofacial development, demonstrating that ablation of Pdgfrb in the NCC lineage results in increased nasal septum width, delayed palatal shelf development and subepidermal blebbing. These defects stem, at least in part, from decreased proliferation of the facial mesenchyme past mid-gestation. Further, we showed that PDGFRalpha and PDGFRbeta genetically and physically interact in the craniofacial mesenchyme to form functional heterodimers with unique signaling properties, thus uncovering a unique mode of signaling for the PDGF family during vertebrate development. Our goal is to characterize novel intracellular pathways and cellular processes engaged downstream of PDGFR induction.
Research in the lab falls under three interrelated themes, with each project combining acute signaling studies in in vitro cell culture models together with developmental analysis of the roles of the PDGFRs and associated signaling molecules and effector proteins in the mouse embryo in vivo:
1. Generating tools to study the dynamics and roles of phosphoproteins active during craniofacial development
2. Exploring how biological specificity is introduced to generate unique responses downstream of PDGFRalpha versus PDGFRbeta engagement
Projects:
* Examining the dynamics of PDGFR dimer-specific activation, internalization and intracellular trafficking in novel PDGFR-BiFC (bimolecular fluorescence complementation) cell lines in vitro
* Examining the spatiotemporal dynamics of PDGFR dimer-specific formation in vivo in the craniofacial mesenchyme of developing mouse embryos using novel PDGFR-BiFC knock-in alleles
* Characterizing PDGFR dimer-specific interactomes through: a) genetic epistasis experiments between Pdgfra mutant mice and both null or constitutively active alleles of genes encoding signaling molecules that are known to bind the receptors; and b) coupling BiFC with affinity purification and mass spectrometry analysis to identify PDGFR dimer-specific interacting proteins
3. Investigating gene expression regulation downstream of PDGFR signaling in the craniofacial mesenchyme
Projects:
* Identifying Srsf3 protein and RNA interactions in response to PDGFRalpha activation in cultured mouse embryonic palatal mesenchyme cells through mass spectrometry and eCLIP (enhanced crosslinking and immunoprecipitation), respectively
* Characterizing the role of phosphorylation of Srsf3 during mouse craniofacial development in vivo using a novel phosphomutant knock-in allele
* Assessing reciprocal signaling between the ectoderm and NCC-derived mesenchyme mediated by Srsf3 via RNA-sequencing of conditional knock-out mouse models
Each of these projects will provide significant insight into the mechanisms underlying mammalian craniofacial development and will ideally lead to new therapeutic directions for the treatment of human craniofacial birth defects such as cleft lip and palate.
Research in the lab falls under three interrelated themes, with each project combining acute signaling studies in in vitro cell culture models together with developmental analysis of the roles of the PDGFRs and associated signaling molecules and effector proteins in the mouse embryo in vivo:
1. Generating tools to study the dynamics and roles of phosphoproteins active during craniofacial development
2. Exploring how biological specificity is introduced to generate unique responses downstream of PDGFRalpha versus PDGFRbeta engagement
Projects:
* Examining the dynamics of PDGFR dimer-specific activation, internalization and intracellular trafficking in novel PDGFR-BiFC (bimolecular fluorescence complementation) cell lines in vitro
* Examining the spatiotemporal dynamics of PDGFR dimer-specific formation in vivo in the craniofacial mesenchyme of developing mouse embryos using novel PDGFR-BiFC knock-in alleles
* Characterizing PDGFR dimer-specific interactomes through: a) genetic epistasis experiments between Pdgfra mutant mice and both null or constitutively active alleles of genes encoding signaling molecules that are known to bind the receptors; and b) coupling BiFC with affinity purification and mass spectrometry analysis to identify PDGFR dimer-specific interacting proteins
3. Investigating gene expression regulation downstream of PDGFR signaling in the craniofacial mesenchyme
Projects:
* Identifying Srsf3 protein and RNA interactions in response to PDGFRalpha activation in cultured mouse embryonic palatal mesenchyme cells through mass spectrometry and eCLIP (enhanced crosslinking and immunoprecipitation), respectively
* Characterizing the role of phosphorylation of Srsf3 during mouse craniofacial development in vivo using a novel phosphomutant knock-in allele
* Assessing reciprocal signaling between the ectoderm and NCC-derived mesenchyme mediated by Srsf3 via RNA-sequencing of conditional knock-out mouse models
Each of these projects will provide significant insight into the mechanisms underlying mammalian craniofacial development and will ideally lead to new therapeutic directions for the treatment of human craniofacial birth defects such as cleft lip and palate.